With dinosaurs out of the way, mammals had a chance to thrive

For dinosaurs, the end of the world began in fire.
The space rock that stamped a Vermont-sized crater into the Earth 66 million years ago packed a powerful punch. Any animal living within about a thousand miles of the impact zone was probably vaporized, says paleontologist Stephen Brusatte of the University of Edinburgh in Scotland.
“Everything would have been toast.”
But outside of the impact zone, amid the smoking ruins of the battered planet, some survivors emerged.
Life there was no picnic. Wave after wave of life-threatening disasters pummeled the animals that remained, says paleontologist Nicholas Longrich of the University of Bath in England. Earthquakes. Wildfires. Volcanoes. Acid rain. Dust and gunk in the air, blotting out the sun. “It’s this series of biblical plagues,” Longrich says.
With little light, much plant life perished, and entire food webs collapsed. Life would have been like an ancient Hunger Games, with all living creatures as contestants. The odds were not in their favor. From sea to land to lake to sky, animals suffered incredible losses.
“You’re basically losing all the big herbivores, all the big carnivores, apex predators in the oceans, entire guilds — wiped out overnight,” Longrich says. On land, he adds, anything bigger than a beaver went extinct. Just a few places in North America offer a fossil record of the early years after the extinction, he says, but “there’s no evidence for anything over 10 kilos surviving.”
Tyrannosaurus rex, Triceratops, Ankylosaurus and all other nonavian dinosaurs gone.
A lucky few animals managed to cope with the dramatic changes reshaping their environment, Brusatte says. But why exactly some animal groups survived and others bit the dust is still one of paleontology’s biggest mysteries.
New fossil research is now helping scientists peer back through time, offering glimmers of what might have been: How some animals made it through one of the worst extinction events the planet has ever seen — and how mammals, in particular, came to dominate.
Sussing out animals’ survival strategies could offer hints about how animals today might handle a changing climate, Brusatte says. It might even expose the evolutionary drivers that shaped modern life. After the extinction, evolution went wild, he says. The survivors “had a new world to play in — a new world to conquer.”
Cretaceous catastrophe
Near the very end of the Late Cretaceous Epoch, right before the world blew up, one of the largest mammals in North America may have been noshing on bones.
Didelphodon vorax, a honey badger–looking creature with oddly bulbous teeth, was petite by today’s standards — weighing just about five kilograms. But it was no lightweight. “Pound for pound, it had the greatest bite force of any mammal we’ve ever measured,” says paleontologist Gregory Wilson of the University of Washington in Seattle.
Wilson and colleagues estimated Didelphodon’s bite force from the shape of its fossilized skull. The mammal could snap its jaws together with about 50 pounds of force — enough to crush bones and crack shells, the team reported December 8 in Nature Communications.
This fearsome skill wasn’t enough to save it: After the asteroid hit and global disasters descended, Didelphodon went extinct — just like duck-billed dinosaurs and Pteranodon.
The colossal wipeout of Didelphodon and so many others is plain to see in the fossil record. In Montana’s badlands, where Wilson and colleagues hunt for ancient teeth and bones, tributaries of the Missouri River carve steep bluffs into the earth, exposing slabs of sandstone and siltstone rock. Montana is part of the Western Interior, an ancient seaway that once cut a wide aisle through North America from the Gulf of Mexico to the Arctic.
Much of what scientists know about the dino-killing event, called the Cretaceous–Paleogene, or K–Pg, extinction, traces back to this sweeping tract of land. The area has rocks with fossils from before and after the extinction event. “We haven’t found many places in the world like it,” Wilson says. Spain, France and Romania hold a few dinosaur and mammalian fossils from this time period (and a handful of underexplored spots in India and South America may offer more). But so far, the Western Interior is home to the best land-based record scientists have.
In Montana, the rocks capture a snapshot of time from about 2 million years before the extinction to roughly 1.5 million years after. A thin layer of reddish-brown clay marks the before and after of the asteroid’s impact. “It’s a line in the sand, almost literally,” Brusatte says. Within the clay, here and elsewhere in the world, scientists find elevated levels of iridium, a silvery-white metal carried to Earth via asteroid. Though not visible by eye (scientists need chemical tests to spot it), the metallic dust marks a memory of the impact known as Chicxulub.
All around the globe, Brusatte says, scientists see “a knife-edge separation in the rock” before and after Chicxulub hit. “For over 150 million years you have tons and tons of dinosaur bones, and then literally — Bam! There’s nothing.”
Dinosaurs were among the animal groups hit hardest by the extinction. Others suffered fewer casualties. In what is now northeastern Montana, about half of fish species survived, Wilson reported at an Origins Project workshop at Arizona State University in 2015. Turtles and salamanders seemed to fare the best, losing only roughly a quarter of their species, Wilson and colleagues reported in a series of studies in 2014.
“Most people think that mammals did awesome,” Wilson says. But at least 75 percent of mammals were snuffed out, according to his analysis, which compared fossils present before and after the extinction. Longrich and colleagues put the number even higher: Of 59 mammalian species living in North America during the Late Cretaceous Epoch, about 93 percent died out after the asteroid hit. Those calculations appeared in the Journal of Evolutionary Biology in August 2016.
Still, some species found a way to endure.
Survival strategies
A small body. An aquatic lifestyle. Night vision. An unfussy palate. Any one of these features could have helped survivors withstand the relentless undoing of their ecosystems.
It makes sense. Small animals would have required less food than large ones and may have had an easier time finding shelter. Animals that lived in water could have been buffered from dramatic temperature swings.
Nocturnal animals would have been able to hunt for food when debris-filled skies wrapped the world in gloom. The right diet, in fact, could have been one of the biggest tickets to survival. Among insects, for instance, the difference between survival and demise depended on dietary diversity.
Some insects are adventurous eaters: They feed on lots of different kinds of plants. Other insects are pickier. Leaf miners, for example, typically dine on just one plant species, or a few closely related ones, which made it hard to survive the cataclysm.
These insects burrow through leaves, leaving behind a distinctive trail. Cataloging the trails and other damage patterns on fossil leaves can give researchers a rough idea of the kinds of insects that went extinct — or survived, says Penn State paleontologist Michael Donovan. It’s like a calling card stamped into stone.
Donovan examined 3,646 fossil leaves found in Patagonia, Argentina, from slices of time bracketing the Chicxulub impact. The leaf-mining patterns seen before the impact vanished after the asteroid hit, he and colleagues reported in Nature Ecology & Evolution in 2016.
That suggests a major extinction of leaf-mining insects, a find echoed in previous results from North Dakota. (Though not all perished. Donovan saw new leaf-mining patterns after the extinction.) Other types of leaf damage did persist through the extinction event — damage made by insects that eat many plant species. Unlike leaf miners, these insects took what they could get in the dark days after the impact. “That’s probably a good way to survive,” Donovan says.
This type of strategy may have helped some species adapt to their new habitat, Longrich says, which after the K–Pg extinction “happened to be this post-apocalyptic wasteland world.” It’s like Mad Max of the movies, he says. “A guy who’s super versatile — good at many different things,” Longrich says, “that’s who’s likely to live through an apocalypse.”
Some animals may have already been plugged into the right food chain. When dinosaurs began dying and leaves fell from trees, the bodies and detritus would have littered the ground and washed into rivers and lakes. That would have been a bonanza for the garbage disposal crew. Decaying matter could feed microbes and fish and insects, which could then feed larger animals, like crocodiles and mammals.
Birdlike dinosaurs with beaks could have cracked into another Cretaceous leftover: seeds. The calorie-rich food could have lasted for decades, says paleontologist Derek Larson of the Philip J. Currie Dinosaur Museum in Alberta and the University of Toronto. Other birdlike dinosaurs, with sharp teeth but no beaks, would have had trouble eating seeds. That might explain why they succumbed, while their close relatives — ancestors of modern birds — survived, he and colleagues suggested last year in Current Biology (SN: 5/14/16, p. 11).
Making it as a mammal
Mammals seemed to capitalize on the detritus-based food chain too, Wilson says. He and University of Washington student Stephanie Smith studied fossils found in northeastern Montana from a 1.2-million-year window after the impact. “Fossil mammals are mostly just teeth,” Smith said at the 2016 Society of Vertebrate Paleontology meeting in Salt Lake City. “Luckily, teeth contain a lot of information.”
Smith compared the intricate details of fossil teeth with those from living mammals to learn about the ancient animals’ diets. In Montana, at least, mammals that lived during the first 200,000 years after the extinction event tended to have teeth that were good for crunching insects — “sharp and pointy,” Wilson says. These animals would have had a reliable source of supper. But plant eaters, which have teeth with big basins for grinding and crushing, would have seen their food supplies wither.
For some mammals, a sharp sense of smell could also have offered a competitive edge. Onychodectes tisonensis, a bull dog–sized mammal that lived about 350,000 years after the extinction, had one of the largest olfactory bulbs of any mammal (relative to the cerebrum) — bigger than those found in even expert sniffers like modern dogs and pigs. The smell organs look like two almonds sticking out from the front of the brain, says James Napoli of Brown University in Providence, R.I., who reported the results at the paleontology meeting last year. He and colleagues built a digital model based on a CT scan of an Onychodectes skull unearthed in New Mexico in 1892.
Having big olfactory bulbs means the animal would have been good at nosing out meals, a valuable skill when food is scarce, Napoli says.
Onychodectes belongs to a weird group of mammals called taeniodonts, says study coauthor Thomas Williamson of the New Mexico Museum of Natural History and Science in Albuquerque. “They have bizarre-looking skulls, enlarged forearms, big claws,” he says. The animals may have survived by digging up and eating tough roots and tubers. “We call them the pigs of the Paleocene.”
Paleontologists don’t know for sure if this group of animals lived through the asteroid crash, or if they arose afterward. There’s just one reported taeniodont fossil from the Late Cretaceous — a partial skull from Alberta, Canada.
If taeniodonts did make it through the impact and its aftermath, an aptitude for rooting out hidden food caches would have been useful. If, instead, the animal group emerged later, Onychodectes could have been one of the early examples of mammalian experimentation.
For more than 150 million years, mammals had been “kept under the thumb of the dinosaurs,” Wilson says. After the extinction, with dinosaurs out of the picture, the “Age of Mammals” could begin.
Boomtime for mammals
In the years after the impact, the world was like a school playground that had banished the big kids.
The animals that survived the early hard years gave rise to a slew of new species able to fill the niches left behind by dinosaurs — and all the other creatures that didn’t make it. Before the impact, humans’ ancestors mostly scurried along the ground. But afterward, with fewer predators and competitors, they were free to try out new lifestyles, like living in trees and gliding.
Placental mammals, a group that includes humans, elephants and most mammals living today, experienced a big evolutionary boom, says Thomas Halliday, a paleobiologist at University College London. “Diversification exploded.”
Without dinosaurs breathing down their necks and with fewer competitors, placental mammals had “freedom to evolve in a variety of new directions,” Halliday says. It’s like they were “exploring almost every aspect of the ways of being a mammal.”
When exactly these mammals arose and how much dinosaurs were holding them back remains controversial: Molecular evidence places their origin tens of millions of years before the dinosaurs died. Fossil evidence puts it closer to the K–Pg extinction.
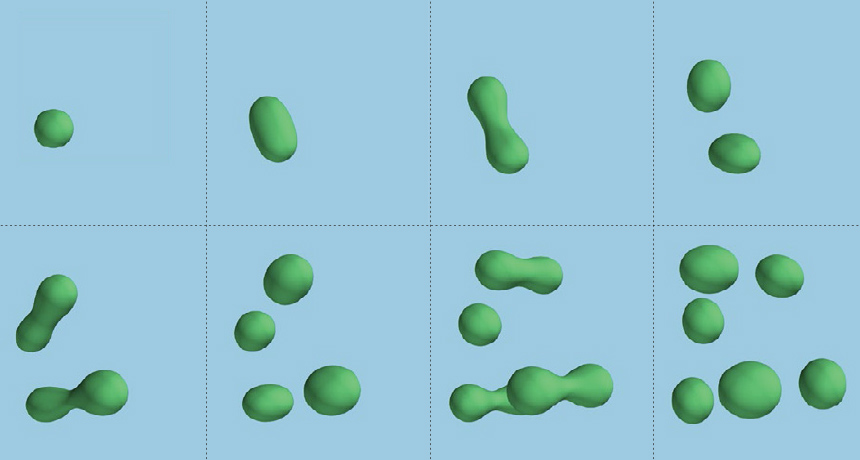
In a series of papers published in 2015 and 2016, Halliday and colleagues analyzed mammalian fossils to sketch out a clearer picture of placental mammals’ history. First, the team built a family tree focused on placental mammals that lived in the Paleocene, the 10-million-year epoch immediately following the extinction. That’s no easy feat, Halliday says, because these animals tend to lack the kind of standout features that would clearly label them as members of one group or another.
So he and colleagues created an exhaustive catalog of 680 body features (such as skull length, tooth number and molar shape) in 177 genera of extinct and living placental mammals and their close relatives. Presumably, animals that shared features were more closely related than those that didn’t. With so many species, the web of potential relationships was astronomical, Halliday says. “There were more possible arrangements … than there are hydrogen atoms in the universe.” The team plugged the data into a computer, which chugged through all the possibilities and came up with the most likely family tree.
Then, the researchers used the tree to calculate rates of evolution. Placental mammals, they found, probably did originate in the Late Cretaceous, but they evolved three times faster after the extinction event than in the 80 million years before it. “We’re talking about new anatomical innovations,” Halliday says: molars good for grinding leaves, limbs adapted for climbing or swimming.
One of these early innovators was Periptychus carinidens, a muscular animal that walked like a bear and had five toes with “weird little hooves,” says University of Edinburgh paleontologist Sarah Shelley. “It’s not like anything alive today.”
Shelley, Williamson and Brusatte described Periptychus fossils found in New Mexico’s San Juan Basin at the 2016 paleontology meeting. “They have really strange cheek teeth,” Williamson says. The teeth are enlarged and conical with big ridges that run from the base to the tip. He thinks Periptychus used its weird chompers to eat hard objects — seeds, perhaps, or unripe fruit.
Periptychus was among the first plant-eating placental mammals to emerge after the extinction — and for a few million years it flourished. Fossils of the animal have been found from West Texas to eastern Montana, Williamson says. “It must have been a highly successful mammal.” But Periptychus couldn’t cope with changes that came later — it died out about 60 million years ago. The animals “were early experiments,” he says, “but they were ultimately dead ends.”
That’s how it goes with evolution, Halliday says. After the dinosaurs died and mammals tested out different modes of life, some found success and others fizzled. “The most successful strategies are honed and the less successful ones are pared away,” he says.
What’s left is what we have today: more than 5,400 different mammal species spread across the world. But descending from an evolutionary winner doesn’t guarantee a safe future. As species carve out an ever more ideal niche, they become more and more vulnerable to extinction, Halliday says. Animals built for a narrow mode of living tend to have a hard time handling disruptions to their environment. And as the climate changes, some species have already begun to suffer. “In the metaphorical sense, we are in the middle of the asteroid strike right now,” he says.
Already, a changing climate has erased pockets of plants and animals across the globe, John Wiens of the University of Arizona in Tucson reported in December 2016 in PLOS Biology. Further warming in coming decades could ramp up extinctions, he warns.
That’s why studying life and death 66 million years ago is still relevant today, Brusatte says. “It’s not just storytelling about the ancient past,” he says. “It can help us understand our modern world,” and maybe even influence conservation strategies to mitigate some of the changes that are happening now.